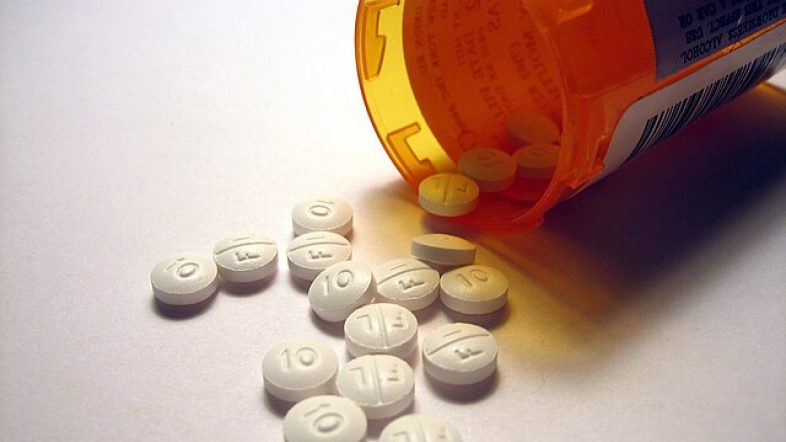
The FDA has now green-lighted a new anti-viral drug from pharmaceutical giant Merck to help fight the coronavirus.
Top Stories
- Biden Admin’s New Report On Gas Exports Basically Undermines One Of Its Key Conclusions December 22, 2024
- Trump’s Border Czar Has Plan For ‘Super’ Sanctuary County Wanting To Shield Its Criminal Migrants From ICE December 25, 2024
- Dem Mayor Facing Biden DOJ Indictment Says He’s ‘Looking Forward’ To ‘Partnering’ With Trump Admin December 24, 2024
- Why Real Republicans Don’t Like Romney December 22, 2024
- Navy Admits DOD Will Shell Out Funds For Obscure Environmental Initiative With No Impact On Military Readiness December 23, 2024
Top Videos
Get curated news content to your inbox
December 23, 2021 6:16 pm - American Briefing News, Coronavirus, FDA, Health, Health Care, Merck, Public Health
5 min. read
FDA Gives Green Light to Merck’s New Anti-Viral Pill to Treat Covid-19
mlance
December 25, 2024 10:47 pm - American Briefing News
Trump’s Border Czar Has Plan For ‘Super’ Sanctuary County Wanting To Shield Its Criminal Migrants From ICE
Tom Homan, the incoming border czar for the Trump administration, has a plan to deal with a major jurisdiction that recently doubled down on a statewide sanctuary law. Earlier in December, the Democrat-controlled San Diego County Board of Supervisors voted to expand on a California sanctuary policy that…
mlance
December 25, 2024 10:46 pm - American Briefing News
5 Times ‘The Breakfast Club’ Callers Clashed With Charlamagne As He Defended Harris
Callers on the popular hip-hop radio show “The Breakfast Club” frequently criticized Vice President Kamala Harris during her brief 2024 presidential campaign, prompting host Charlamagne Tha God to defend her. Harris did not conduct a sit-down interview for over a month after launching her campaign and largely …
mlance
December 25, 2024 10:44 pm - American Briefing News
Army That Helped America Defeat ISIS Finds Itself At War With … Another US Ally

Soldiers of the Somali National Army (SNA) on parade, during a pass-out ceremony for Somali National Army (SNA) held in Baidoa , Somalia on April 07, 2015. AMISOM Photo / Sabir Olad.
An increasingly complex conflict is emerging in the ruins of Bashar al-Assad’s fallen Syrian regime, pitting an American-backed force against a proxy that’s funded by a major U.S. ally. The Syrian National Army (SNA), backed by NATO member Turkey, is locked into a conflict in northern Syria with the U.S.-backed…
mlance
December 24, 2024 11:39 pm - American Briefing News
Dem Mayor Facing Biden DOJ Indictment Says He’s ‘Looking Forward’ To ‘Partnering’ With Trump Admin
Democratic New York City Mayor Eric Adams on Monday disclosed what excites him most regarding working with President-elect Donald Trump’s upcoming administration. Adams, who is notably facing a federal indictment, recently met with Trump’s incoming border czar Tom Homan, and said they agreed…
mlance
December 24, 2024 11:37 pm - American Briefing News
‘Gnashing Of Teeth’: Guess Which Political Party Is Dreading Holiday Celebrations With People They Disagree With?
Democrats appear to have less holiday cheer than their conservative counterparts this Christmas, a newly reported Axios-Ipsos poll found. Over 61% of Democrats said time with their loved ones over the holidays makes them more stressed out, while just 39% of Republicans said the same, according to the…
mlance
December 24, 2024 11:26 pm - American Briefing News
Biden Admin Quietly Scraps Proposal That Would Force More Employers To Cover Employees’ Birth Control
The Biden administration quietly withdrew a proposal to reduce the ability of employers to opt-out of covering birth control for employees on Monday. The Department of Health and Human Services (HHS) published notice in the Federal Register on Monday that the agency is pulling back its…
mlance
December 23, 2024 11:39 pm - American Briefing News
‘Depraved’: Man Accused Of Burning Woman Alive On Subway Is Previously Deported Illegal Migrant, ICE Confirms
The man accused of burning a sleeping subway passenger to death is an illegal migrant who was previously deported from the United States, federal immigration authorities have confirmed. The New York Police Department has detained an illegal Guatemalan migrant who they suspect is responsible for burning a woman…
mlance
December 23, 2024 11:33 pm - American Briefing News
Victims’ Families And Friends Slam Biden For ‘Heartless Decision’ To Shield Child Murderer, Cop Killer From Death Row
Victims’ friends and family members condemned President Joe Biden’s decision Monday to commute the death sentences of criminals who murdered their loved ones. Biden granted clemency to 37 men on death row convicted for brutal murders, including of children, reclassifying their sentences to life without parole. Kathleen Zellner, an attorney who represented…
mlance
December 23, 2024 11:32 pm - American Briefing News
Radio Silence From Kia Continues After DCNF Exposed Automaker’s Ties To Nonprofit Pushing Trans Books On Kids
Car manufacturer Kia has continued to stonewall following a Daily Caller News Foundation report on the company’s ties to a nonprofit that distributes LGBTQ-themed books to children. The Gay, Lesbian and Straight Education Network’s (GLSEN) Rainbow Library — a program that provides teachers with…
mlance
December 23, 2024 2:23 am - American Briefing News
Biden Spends Waning Days Of Presidency Dishing Out $1,000,000,000 To Preserve Amazon Rainforest
President Joe Biden’s administration is dedicating $1 billion to preserve the Ecuadorian Amazon as a means of addressing climate change, White House press secretary Karine Jean-Pierre announced Friday. The president traveled to the Amazon Rainforest in mid-November to announce the United States’ pledge to increase U.S. international climate finance to over…
mlance
December 23, 2024 2:21 am - American Briefing News
Trump Made TikTok Great Again
In today’s digital era, where social media platforms serve as the battlegrounds for ideas, information and cultural exchange, the conversation around banning TikTok must be approached with caution and a deep understanding of its implications. With over 170 million American users, TikTok has transcended mere entertainment…
mlance
December 23, 2024 2:17 am - American Briefing News
Navy Admits DOD Will Shell Out Funds For Obscure Environmental Initiative With No Impact On Military Readiness

100726-6720T-N-086
EAST SEA (July 26, 2010) U.S. Navy and Republic of Korea ships transit the East Sea in a 13-ship formation. The Republic of Korea and the United States are conducting the combined alliance maritime and air readiness exercise "Invincible Spirit" in the seas east of the Korean peninsula from July 25-28, 2010. This is the first in a series of joint military exercises that will occur over the coming months in the East and West Seas. (U.S. Navy photo by Mass Communication Specialist 3rd Class Adam K. Thomas/Released)
A little-known environmental provision in the fiscal year 2025 National Defense Authorization Act (NDAA) has no impact on military operations, but will instead serve to “protect the native vegetation,” a Navy spokesperson told the Daily Caller News Foundation in a statement Tuesday. The nearly $884 billion defense …
mlance
December 22, 2024 1:48 am - American Briefing News
Lame Duck Biden Admin Faces Mounting Pressure To Shower Anti-Israel, Anti-Cop Nonprofit With Taxpayer Cash
The Biden administration is facing mounting pressure to dole out tens of millions of taxpayer dollars to a left-wing nonprofit that advocates for defunding the police, prison abolition and the “liberation” of Palestinian territories before President-elect Donald Trump takes office. The left-wing advocates lobbying President Joe…
mlance
December 22, 2024 1:47 am - American Briefing News
Why Real Republicans Don’t Like Romney
Republican Utah Sen. Mitt Romney became the first, and for a time, the only senator in U.S. history to vote to convict an impeached president of his own party. That vote followed the first impeachment of President Donald Trump over the allegation that he solicited Ukraine to interfere in the…
mlance
December 22, 2024 1:10 am - American Briefing News
Biden Admin’s New Report On Gas Exports Basically Undermines One Of Its Key Conclusions
Available data and recent history contradict one of the key points of the Biden administration’s long-awaited study on liquefied natural gas (LNG) exports and Energy Secretary Jennifer Granholm’s attempt to spin the study into something it is not. The Department of Energy (DOE) released the study on Tuesday,…
mlance
December 21, 2024 12:20 am - American Briefing News
Fmr FBI Asst Director Warns Terrorists Are ‘Well Embedded’ In US, Says Alert Should Be ‘Higher’ Than Current Level
Former FBI Assistant Director Chris Swecker warned Friday on CNN that terrorists are “well embedded” within the United States, stating the threat level should be “higher” following an attack in Germany. A 50-year-old Saudi doctor allegedly drove his car into a crowded Christmas market in Magdeburg, Germany on…
mlance
December 21, 2024 12:16 am - American Briefing News
Biden Admin Makes Last Ditch Attempt To Stymie Trump’s Plans
The Biden Administration will no longer pursue efforts to rewrite Title IX to include gender identity amid numerous legal challenges. President Joe Biden first proposed revisions to Title IX, which prohibits discrimination based on sex in public schools receiving federal funding, in 2022 and released a finalized version…
mlance
December 21, 2024 12:14 am - American Briefing News
‘Isn’t Just Germany’s Problem’: Fmr Trump Adviser Warns Sanctuary Cities Could Face Same Terror Threats As Germany
Former State department senior adviser Christian Whiton warned Friday of potential terror threats that sanctuary cities in the United States could face. During an appearance on Laura Ingraham’s “The Ingraham Angle,” Whiton discussed the driver who plowed into the crowd at a Christmas Market in Germany. Whiton criticized…
mlance
December 20, 2024 1:43 am - American Briefing News
NYT’s Maggie Haberman Admits Biden’s Low Profile May Have Fueled Chaos, Says Govt ‘Bloat’ Hard To ‘Argue’ Against
New York Times reporter Maggie Haberman admitted Thursday on CNN that President Joe Biden’s lack of visibility may be contributing to the chaos in Congress over the spending bill, stating it’s hard to “argue” with the government’s “bloat” in funds. While Biden has remained notably out of the public spotlight…
mlance
December 20, 2024 1:14 am - American Briefing News
Trump And Key European Leader Say They’ll Pursue Fair And Sustainable Peace Efforts For Ukraine
German Chancellor Olaf Scholz and President-elect Donald Trump pledged to expedite efforts to achieving a “fair, just, and sustainable peace” in Ukraine. While attending the European Council summit in Brussels, Scholz engaged with Trump via phone, according to German government spokesperson Steffen Hebestreit, as reported by Politico. The…
mlance
December 20, 2024 1:05 am - American Briefing News
Trump-Endorsed Plan To Avert Government Shutdown Fails In House Vote
House lawmakers rejected Speaker of the House Mike Johnson’s new stopgap funding bill 233-174 Thursday evening. 38 Republicans, including Republican Rep. Chip Roy of Texas, voted against the new continuing resolution (CR), citing their opposition to increasing the debt ceiling without significant spending cuts and allowing $110 billion in disaster…
mlance
December 18, 2024 11:49 pm - American Briefing News
‘Mistakes Were Made’: Dem Senator Explains Why He Thinks His Party Got Their ‘A*ses Kicked’
Democratic Hawaii Sen. Brian Schatz detailed on a podcast Wednesday how he believes that since 2020 the Democratic Party’s rhetoric has led them to get their “asses kicked” in November, losing the House, the Senate, and the White House. Following President-elect Trump’s win in November, Democrats began to …
mlance
December 18, 2024 11:48 pm - American Briefing News
As Fears Mount Over Mysterious Drones, Biden Admin Sticks To Its Script: Nothing To See Here
Unidentified drones are in the air across large swaths of the country, but Americans nationwide are in the dark as to who is controlling them and for what purpose. Americans started reporting mysterious drone sightings in New Jersey in mid-November, with more sightings reported in the weeks since…
mlance
December 18, 2024 11:47 pm - American Briefing News
Make The Military Care About Being Lethal Again

Secretary of Health Dr. Rachel Levine speaking at the virtual press conference. The Pennsylvania Department of Health today confirmed as of 12:00 a.m., March 20, that there are 83 additional positive cases of COVID-19 reported, bringing the statewide total to 268. County-specific information and a statewide map are available here. All people are either in isolation at home or being treated at the hospital. Harrisburg, PA- March 20, 2020
It is estimated that fewer than 23% of Americans between 17 and 24 are qualified to serve in the U.S. military. The reasons range from obesity and drug use to mental health issues. Today, only a small percentage of our nation has served in…
mlance
December 17, 2024 11:20 pm - American Briefing News
‘Election Interference’: Fed Leaves Trump With Parting Gift After Conveniently Cutting Rates Right Before Election
After slashing interest rates just weeks out from the November presidential election, the Federal Reserve is now leaving an inflationary “ticking time bomb” for the incoming Trump administration to defuse. Price increases accelerated in October and November following the Fed’s decision to reduce interest…
mlance
December 17, 2024 11:19 pm - American Briefing News
Illegal Immigrant On Terror Watchlist Arrested After Release From Detention
United States Border Patrol (USPB) Chief Jason Owens confirmed Tuesday on social media that the agency arrested a South African national “suspected of terror” in Brooklyn, N.Y. In a post to X, Owens said USPB agents arrested the unidentified South African national. Owens also posted a picture of…
mlance
December 17, 2024 11:11 pm - American Briefing News
‘A Dumpster Fire’: Conservative Lawmakers Slam ‘Fundamentally Unserious’ Spending Bill Negotiated By House Leadership
Conservative lawmakers are slamming House Republican leadership following the release of a massive spending bill that will fund the government through mid-March to avert a partial government shutdown by the Friday night deadline. The sprawling 1,574-page bill, known as a continuing resolution (CR), will temporarily extend current government funding levels…
mlance
December 16, 2024 11:07 pm - American Briefing News
Judge Merchan Won’t Budge On Throwing Out Trump Conviction, Despite U.S. Supreme Court Ruling
New York Judge Juan Merchan ruled Monday that President-elect Donald Trump’s felony conviction in his hush money case should not be thrown out due to the U.S. Supreme Court’s ruling on his presidential immunity, according to a court filing. Merchan issued his decision to reject Trump’s lawyers’ attempt to dismiss…
mlance
December 16, 2024 11:06 pm - American Briefing News
Bill Clinton Warns Of ‘War’ On ‘Diversity’ In American Politics
Former President Bill Clinton warned on a podcast Monday that he believes there’s a “war” on “diversity” within American politics following President-elect Donald Trump’s win in November. During Vice President Kamala Harris’ campaign, Clinton publicly supported her, joining the trail in key battleground states in October. On the…
mlance
December 16, 2024 11:05 pm - American Briefing News
Criminal Defense Attorney Predicts Mangione’s Extradition Move, Reveals Potential Jury’s ‘Real Issue’
Criminal defense attorney Ben Brafman predicted Monday on CNN that alleged UnitedHealthcare assassin Luigi Mangione will waive his extradition rights, later revealing that the “real issue” with a New York City jury will be finding people who don’t support his case. Mangione was arrested on Dec. 9 in…
mlance
December 16, 2024 1:17 am - American Briefing News
House Republicans Finally Putting The Squeeze On Bloated Pentagon Budget
This week the House of Representatives slammed the door on bigger defense budgets without more accountability. The fissure opened at the end of the 118th Congress among Republicans voting on funding for the U.S. military. After much wrangling, the Senate and House agreed to setting the Pentagon’s budget in fiscal 2025…
mlance
December 16, 2024 1:16 am - American Briefing News
‘Party Of The Working Class’: Trump Critic Mitt Romney Credits Trump For Reshaping GOP As He Exits Politics
Republican Utah Sen. Mitt Romney, a longtime critic of President-elect Donald Trump, gave Trump “credit” Sunday on CNN for successfully transforming the Republican Party with the Make America Great Again (MAGA) movement. For years, Romney has been vocal in his criticism of Trump, writing an op-ed in 2019…
mlance
December 16, 2024 1:15 am - American Briefing News
Stripper Who Falsely Accused Duke Lacrosse Team Of Vicious Gang Rape Finally Admits She Lied
The stripper who falsely claimed that members of the Duke University men’s lacrosse team savagely raped her in 2006 finally admitted Thursday that she made up the allegations. Krystal Mangum, the exotic dancer behind the allegations, admitted that she “testified falsely” that she was raped by David Evans, Collin Finnerty…
mlance
December 15, 2024 1:21 am - American Briefing News
EXCLUSIVE: ‘Humorless Pronoun Robots’: Tucker Carlson Gives Corporate America, Zyn ‘A Middle Finger’ Through Alp Launch
Daily Caller News Foundation co-founder Tucker Carlson was joined by friends and supporters Thursday evening outside of Nashville, Tennessee at the launch of his new nicotine pouch line, ALP, where he explained how Zyn’s attack on him led to the creation of his rival product. Carlson announced the launch of…
mlance
December 15, 2024 1:19 am - American Briefing News
Biden Admin Dumped Hundreds Of Thousands Into Ensuring Military Parents Affirm Their Child’s Gender Identity
The Biden-Harris administration awarded a federal grant to facilitate training for military families that pushes parents to affirm their child’s self-selected gender identity. The $850,000 grant project, known as Centerstone’s LGBTQI+ Family Support Program (Family+), intends to provide “affirming interventions for 230 youth and their families” and train 100 staff…
mlance
December 15, 2024 1:18 am - American Briefing News
DOGE Must Focus On Big Picture To Achieve Big Change
President-elect Donald Trump’s new Department of Government Efficiency (DOGE) is wasting no time in laying the groundwork for its effort to cut the size and scope of government. That Elon Musk and Vivek Ramaswamy are the right men to lead this effort…
mlance
December 14, 2024 1:47 am - American Briefing News
New Jersey Mayor Says First Responders Told To Call Bomb Squad, Wear Hazmat Suits If Drone Is Discovered
A New Jersey mayor told Fox News host Harris Faulkner Friday that first responders were told to contact the bomb squad and wear hazmat suits if they encountered one of the mysterious drones. The drones have appeared over the sky of New Jersey, in some cases flying in formation, in recent…
mlance
December 14, 2024 1:45 am - American Briefing News
‘The View’ Co-Host Ponders Whether Trump Is Really So Extreme After All
“The View” co-host Sara Haines pondered Friday whether President-elect Donald Trump truly has extreme positions on major policy issues following his interview with Time Magazine. Trump, who was named “Person of the Year” on Thursday, told the magazine the transgender bathroom issue only affects “a small number of…
mlance
December 14, 2024 1:43 am - American Briefing News
‘Source Of Profound Regret’: Firm Pays Half Billion Settlement To Avoid Criminal Prosecution For Fueling Opioid Crisis
A consulting giant that helped fuel the United States’ deadly opioid epidemic agreed to pay a massive settlement to avoid criminal prosecution, according to court papers filed Friday. McKinsey & Company, an international management consulting firm that advised Purdue Pharma to “turbocharge” sales of Oxycontin during the height…
mlance
December 13, 2024 2:16 am - American Briefing News
Daniel Penny’s ‘Crime’? Wrong Race, Wrong Place
The same day Daniel Penny, the retired Marine and architecture student, pleaded not guilty to manslaughter and negligent homicide, a New York grand jury declined to recommend charges against Jordan Williams. Williams and his girlfriend, who are both black, were on a Brooklyn subway train when…
mlance
December 13, 2024 2:15 am - American Briefing News
Jonathan Turley Questions Why J6 FBI Informants Weren’t Charged While 1,500 Others Were Arrested
George Washington University law professor Jonathan Turley, following the release of a new report, said Thursday that there were “more questions than answers” about FBI informants at the Jan. 6, 2021 riot at the Capitol building. Hundreds of people stormed the Capitol building during the certification of the electoral votes on…
mlance
December 13, 2024 2:14 am - American Briefing News
Biden Official Signals Support For Two Of RFK’s Signature Issues On His Way Out The Door
A Biden official appears to be signaling support for key parts of Robert F. Kennedy’s Make America Healthy Again agenda on his way out the door. Food and Drug Administration (FDA) Commissioner Robert Califf voiced support for several MAHA priorities to incentivize healthy eating and eliminate harmful ingredients…
mlance
December 11, 2024 9:43 pm - American Briefing News
‘Social Media Is Now The Jury’: Kevin O’Leary Says Health Execs Need To ‘Read The Room,’ Warns Public Has Turned
“Shark Tank” co-star Kevin O’Leary said Wednesday evening on CNN that health care executives need to “read the room” as public appeal has turned against them following the shooting of United Healthcare CEO Brian Thompson. Public appeal for Thompson’s alleged assassin, 26-year-old Luigi Mangione, has been driven by people online,…
mlance
December 11, 2024 9:42 pm - American Briefing News
Trump Seeks Dismissal Of Defamation Case Tied To Central Park Five Allegations
President-elect Donald Trump requested Wednesday that a federal court drop a defamation lawsuit against him tied to the Central Park Five allegations. Trump’s attorneys say his remarks about the Central Park Five—five wrongly convicted men of Black and Hispanic descent— are protected as free speech, according to Reuters.
mlance
December 11, 2024 9:41 pm - American Briefing News
Trump’s Reported Plan For ICE Leaves Nowhere For Criminal Migrants To Hide

ICE arrests criminal aliens as part of a 3-day targeted enforcement operation in North Texas and Oklahoma.
The incoming Trump administration intends to scrap a longstanding policy that largely prevents federal immigration authorities from arresting illegal migrants in areas that are known as “sensitive” locations, according to an NBC News report. President-elect Donald Trump would like to allow Immigration and Customs Enforcement (ICE) agents to begin arresting…
mlance
December 11, 2024 1:24 am - American Briefing News
Daniel Penny Stands By His Actions, Says He’d Face Court ‘Million’ Times To Save Others
Former Marine Daniel Penny said Tuesday that he stands by his actions on a New York City subway, despite enduring years of harsh criticism. During an interview with Judge Jeanine Pirro on “The Five,” Penny revealed his motivations and said he intervened to prevent potential harm as he…
mlance
December 11, 2024 1:21 am - American Briefing News
Ukraine Sent Drone Operators And Drones To U.S. Designated Terrorist Group
Ukraine reportedly deployed drone operators and drones Tuesday to a group that the U.S. has designated as a terrorist organization. The Syrian rebels who swept to power in Damascus last weekend reportedly received drones and other support from Ukrainian intelligence operatives who sought to undermine Russia and its Syrian allies,…
mlance
December 11, 2024 1:20 am - American Briefing News
US Treasury Turns Frozen Russian Assets Into $20 Billion Loan For Ukraine
The U.S. Treasury Department on Tuesday executed the transfer of its $20 billion share of a massive $50 billion G7 loan to Ukraine. The funds, routed through a World Bank intermediary called the Facilitation of Resources to Invest in Strengthening Ukraine Financial Intermediary Fund (FORTIS Ukraine FIF), aim to bolster…
mlance
December 9, 2024 9:57 pm - American Briefing News
Biden Cabinet Secretary Breaks Down In Tears While Presenting Biden With Blanket
Secretary of the Interior Deb Haaland was seen in tears as she introduced President Joe Biden at his final Tribal Nations Summit on Monday, as she presented him with a blanket. Biden and officials from his administration attended the White House’s Tribal Nations Summit, giving tribal leaders the…
mlance
December 9, 2024 9:55 pm - American Briefing News
TikTok Owner Pleads For Legal Relief Against US TikTok Ban
ByteDance, the Chinese conglomerate that owns TikTok, approached an appeals court Monday to temporarily halt a ruling that mandates the company either sell TikTok or face a ban in the United States by Jan. 19. ByteDance sought an injunction from the U.S. Court of Appeals in Washington, D.C., seeking to…
mlance
December 9, 2024 9:54 pm - American Briefing News
Ernst Signals Support For Hegseth After ‘Encouraging Conversations’
Pete Hegseth appears to have won over a prominent holdout in his quest to serve as President-elect Donald Trump’s Department of Defense secretary. Iowa Republican Sen. Joni Ernst signaled support for Hegseth’s nomination following their meeting Monday afternoon. “I appreciate Pete Hegseth’s responsiveness and respect for the process,” Ernst …
mlance
December 8, 2024 9:01 pm - American Briefing News
Illegal Migrant Accused Of Raping Woman Along Hiking Trail In Sanctuary County Had History Of Prior Arrests
An illegal migrant accused of raping a woman on a popular hiking path in a Washington, D.C.-area suburb previously had been arrested and released multiple times by local law enforcement. A woman walking home on Nov. 18 along the Washington & Old Dominion Railroad Trail in Herndon, Virginia was attacked…
mlance
December 8, 2024 8:56 pm - American Briefing News
‘They Came In Illegally’: Trump Clashes With ‘Meet The Press’ Host Over Deportation Promises
President-elect Donald Trump and “Meet the Press” host Kristen Welker clashed over Trump’s promises to deport illegal immigrants during an interview that aired Sunday morning. During the 2024 presidential campaign, Trump highlighted a series of crimes involving illegal immigrants, including the murders of Rachel Morin, Laken Riley and Jocelyn Nungaray and the …
mlance
December 8, 2024 8:55 pm - American Briefing News
NBC’s Kristen Welker Hounded Trump Over A Dozen Times During Interview About Whether He Would Target His Opponents
“Meet the Press” host Kristen Welker on Sunday asked President-elect Donald Trump more than a dozen times about whether he would use the levers of power to target his political rivals. Trump nominated former Republican Attorney General Pam Bondi of Florida to be attorney general and former Trump administration official Kash…
mlance
December 7, 2024 10:11 pm - American Briefing News
You Might Have Missed It, But Ray Epps’ Lawsuit Against Tucker Went Down In Flames
A federal judge dismissed a January 6th defendant’s defamation lawsuit against Fox News and its former primetime TV anchor, Daily Caller co-founder Tucker Carlson on Wednesday. Delaware Federal District Court Judge, Jennifer Hall, ruled that Carlson’s reporting on Epps was protected under the First Amendment because Epps’ lawyers…
mlance
December 7, 2024 10:10 pm - American Briefing News
Biden Confirms What Conservatives Have Known All Along
After months of insisting he would not pardon his son Hunter, President Joe Biden did just that — as almost anyone other than left-wing cable news legal analysts and Trump-hating late-night comics expected. In doing so, Biden effectively confirmed the Trump “conspiracy theory” of a two-tiered justice system.
mlance
December 7, 2024 10:08 pm - American Briefing News
Biden Admin Cut Big Check To Activists Using Schools As Recruiting Grounds For LGBT ‘Family Therapy’ Program
A Michigan group received federal funds for a “family therapy” program that instructs parents to affirm their child’s gender confusion — and enlists public school districts in the effort. A nearly $1.3 million f_ederal grant awarded in early 2024 to Arbor Circle, a resource center that offers counseling and…
mlance
December 6, 2024 10:54 pm - American Briefing News
‘A Lot Of Failures In One Day’: Seemingly Random Woman Able To Pull Off Movie-Like Move At Airport, On Plane
A 57-year-old Russian national surprised authorities last week by boarding a Delta flight from New York to France without a passport or boarding pass, according to multiple outlets. The suspect, identified as Svetlana Dali, appeared in Brooklyn federal court on Friday and is facing one count of being a stowaway…
mlance
December 6, 2024 10:48 pm - American Briefing News
Reporters Bombard KJP For Answers On Biden’s Pardoning Of Son Despite Months Of Promising It Would Not Happen
Reporters bombarded White House press secretary Karine Jean-Pierre at a Friday briefing about the pardoning of Hunter Biden despite herself and President Joe Biden promising his son would not receive one from his father. The president formally issued a “full and unconditional” pardon for his son on Sunday…
mlance
December 6, 2024 10:47 pm - American Briefing News
It Only Took 12 Months For Roughly 1,000,000 Native-Born Jobs To Vanish Under Biden-Harris

President Joe Biden deliver remarks ceasefire between Israel and Hezbolllah, Tuesday, November 26, 2024, in the Rose Garden of the White House. (Official White House Photo by Adam Schultz)
More than 1,000,000 fewer native-born Americans are employed than last year, according to data from the Bureau of Labor Statistics (BLS). The number of foreign-born workers employed increased by roughly 400,000 year-over-year in November, while 1,094,000 fewer native workers were employed, BLS data shows. The large disparity…
mlance
December 5, 2024 11:44 pm - American Briefing News
It’s Time To Improve Our Ports
Some union leaders are self-destructive idiots. America’s ports have fallen behind. Not a single one ranks in the top 50 worldwide. A big reason is that dock unions stop innovation. This fall, the International Longshoremen’s Association shut down East and Gulf coast ports, striking for a raise and…
mlance
December 5, 2024 11:43 pm - American Briefing News
‘As Long As Donald Trump Wants Me’: Hegseth Vows To Stay As Trump’s Defense Nominee Amid Challenges
Despite doubts among some about his chances, Pete Hegseth said Thursday he intends to stay on as President-elect Donald Trump’s nominee for secretary of Defense. Hegseth told reporters he has no plans to withdraw his nomination, according to Politico. He’s defended himself against numerous accusations. Hegseth was the…
mlance
December 5, 2024 11:39 pm - American Briefing News
GOP Senators Who Could Block Tulsi Gabbard’s Confirmation Took Huge Checks From Defense Industry
Senate GOP hawks who receive large sums from the defense industry could be mobilizing to tank one of President-elect Donald Trump’s national security nominees. Former Democratic Hawaii Rep. Tulsi Gabbard, nominated by Trump to helm the Office of the Director of National Intelligence, could face opposition from Senate…
mlance
December 4, 2024 10:54 pm - American Briefing News
SCOTUS Conservatives Seem Ready To Uphold Child Sex Change Bans — But One Justice Is A Wild Card
Every conservative justice expressed skepticism Wednesday of the Biden administration’s challenge to state ban on child sex changes, except Justice Neil Gorsuch Gorsuch, who authored a landmark ruling in 2020 expanding sex discrimination to include gender identity and sexual orientation in the context of employment, didn’t say a word during over…
mlance
December 4, 2024 10:53 pm - American Briefing News
ISIS-K And Biden’s Border
When President George W. Bush stood in front of the U.S. Capitol on Jan. 20, 2005, and gave his second inaugural address, as this column has noted before, he argued that maintaining freedom in the United States would require spreading freedom all around the world. “We are…
mlance
December 4, 2024 10:50 pm - American Briefing News
Nadler Concedes To Raskin, Bows Out Of Running For Top Position
Another influential House Democrat is stepping down from committee leadership after facing a competitive challenge from a younger member. Democratic New York Rep. Jerry Nadler, ranking member of the House Judiciary Committee, announced his decision to not seek another term as the influential committee’s top Democrat in a …
mlance
December 4, 2024 12:45 am - American Briefing News
Fmr Obama Aide Slams Biden As ‘A Tragic Figure,’ Says His Ego Keeps Getting ‘In The Way’
Former Obama aide Jon Favreau on his show Tuesday called President Joe Biden “a tragic figure,” stating that the president’s ego has consistently gotten “in the way” throughout his term. Following Biden’s announcement that he would pardon his son — despite both him and his staff previously denying he would…
mlance
December 4, 2024 12:44 am - American Briefing News
US Struggles To Remove Chinese Hackers From Major Telecom Networks
Chinese government-backed hackers have infiltrated major U.S. telecom networks, and officials are now struggling to remove their access, U.S. authorities announced Tuesday. Jeff Greene, a senior official at the U.S. Cybersecurity and Infrastructure Security Agency (CISA), pointed out the complexity of the situation, according to CNN. He explained…
mlance
December 4, 2024 12:42 am - American Briefing News
Trump Promised A Ukraine-Russia Peace Deal — Here’s One Plan
As he promised throughout his campaign, one of President-elect Donald Trump’s top priorities is to end the war in Ukraine. For 33 months now, the Biden administration has refused to give Ukraine in timely fashion enough of the long-range capabilities and air defenses it needs to win decisively. As a result,…
mlance
December 2, 2024 10:38 pm - American Briefing News
Anti-Trump Republican Admits Biden’s Pardon Makes It Tougher To Convince Middle America Trump Is ‘A Unique Threat’
Former Republican Illinois Rep. Joe Walsh admitted Monday on a show that President Joe Biden’s pardon of his son makes it tougher to convince middle-ground voters that President-elect Donald Trump is a “unique threat.” Biden released a statement Sunday evening announcing that he pardoned his son, Hunter Biden,…
mlance
December 2, 2024 10:37 pm - American Briefing News
Staffer For Democratic Rep Arrested For Carrying Ammunition Onto Capitol Property
Michael Hopkins, a communications director for Democratic New York Rep. Joe Morelle, was arrested Monday for carrying ammunition inside the Cannon House Office Building. Officials with the United States Capitol Police (USCP) spotted ammunition through an X-ray screening Monday morning. “At approximately 8:45 a.m., a House staffer entered the Cannon…
mlance
December 2, 2024 10:34 pm - American Briefing News
General Motors Strikes Deal To Offload Battery Plant Stake To LG
General Motors (GM) announced Monday a shift in its electric vehicle (EV) strategy by agreeing to sell its share of a nearly completed battery plant in Lansing, Mich. to its joint venture partner, LG Energy Solution of South Korea. GM confirmed that the arrangement with LG is a…
mlance
December 1, 2024 8:43 pm - American Briefing News
Time To Make The US Navy The Fighting Force It Should Be

120626-N-RY232-721 ATLANTIC OCEAN (June 26, 2012) - Guided-missile destroyers USS Jason Dunham (DDG 109) and USS Farragut (DDG 99) sail alongside Nimitz-class aircraft carrier USS Dwight D. Eisenhower (CVN 69) in the Atlantic Ocean, June 26. Dwight D. Eisenhower is on a regularly scheduled deployment in support of Maritime Security Operations (MSO) and Theater Security Cooperation (TSC) efforts in the U.S. 5th and 6th Fleet areas of responsibility. IKE deployed as part of Eisenhower Carrier Strike Group (CSG), which includes CSG 8, IKE, guided-missile cruiser USS Hue City (CG 66), guided-missile destroyer USS Farragut (DDG 99), guided-missile destroyer USS Winston S. Churchill (DDG 81), USS Jason Dunham (DDG 109), the seven squadrons of Carrier Air Wing (CVW) 7, and Destroyer Squadron 28. (U.S. Navy photo by Mass Communication Specialist 2nd Class Julia A. Casper/Released)
There’s an old saying in the U.S. Navy: “If it moves, salute it. If it doesn’t, paint it grey.” President-elect Donald Trump’s pick for secretary of the Navy, John Phelan, announced this week, will get plenty of salutes. As he assumes the helm of the world’s second-largest navy, the savvy…
mlance
December 1, 2024 8:39 pm - American Briefing News
‘Completely Corrupted’: GOP Senator Slams ‘Woefully Inexperienced’ Biden Officials While Backing Trump’s FBI Pick
Republican Tennessee Sen. Bill Hagerty called out the “woefully inexperienced” officials under President Joe Biden’s administration on NBC News Sunday in response to criticism of President-elect Donald Trump’s pick of Kash Patel as FBI director. Pushback from Democrats and other officials against Patel came after Trump …
mlance
December 1, 2024 8:37 pm - American Briefing News
Israel Is Tipping The Scales
It is not supposed to be difficult — there are good guys and bad guys, right? As fighting in Syria is renewed and escalates, it is not actually that simple. In September, the U.N. Commission of Inquiry on Syria noted factional fighting and, as usual, blamed Israel. “Heightened regional tensions…
mlance
December 1, 2024 3:13 am - American Briefing News
‘You Can’t Say You Weren’t Warned’: Five Times CNN’s Harry Enten Was Right About The Election
CNN data reporter Harry Enten told the network’s viewers Oct. 30 that there were signs that President-elect Donald Trump could win the election, remarking at the time, “You can’t say you weren’t warned.” Trump secured the 270 electoral votes necessary to win the presidency early in the morning of Nov. 6,…
mlance
December 1, 2024 3:09 am - American Briefing News
RFK’s Calls To Ban One Of Big Pharma’s Most Powerful Tools Rattle Drugmakers Despite Uncertain Political Prospects
President-elect Donald Trump’s nomination of Robert F. Kennedy Jr. to helm the Department of Health and Human Services(HHS) is reportedly rattling drugmakers in light of Kennedy’s prior calls to ban pharmaceutical advertising. If confirmed by the Senate to serve as HHS secretary, Kennedy could marshal the country’s public health agencies…
mlance
December 1, 2024 3:08 am - American Briefing News
Illegal Immigrants Are Committing Crimes — Here’s The Data
In June, Victor Martinez-Hernandez was charged with the murder of Rachel Morin, a mother of five in Maryland. Police in Oklahoma tracked the accused repeat offender down with a sample of his DNA recovered from a Los Angeles home invasion in which a nine-year-old girl and…
mlance
November 29, 2024 9:45 pm - American Briefing News
The Case for Peter Hegseth — Time To Try Something Different
Success in today’s world favors smart, creative leaders who can quickly adapt and make decisions that benefit their organizations. President-elect Donald Trump’s choice of Pete Hegseth to lead the Department of Defense marks a significant shift from his first administration. Hegseth, with fewer ties to the traditional defense establishment, is…
mlance
November 29, 2024 9:44 pm - American Briefing News
Defeated Dem Senator Claims GOP ‘Rigged The System’ To Win Election
Democratic Sen. Sherrod Brown of Ohio claimed he lost reelection because Republicans “rigged the system” during a heated interview on CNN Thursday. Businessman Bernie Moreno defeated Brown, a three-term senator, Nov. 5 by a 50.2% to 46.6% margin. Brown alleged Republicans “rigged the system” after complaining about ads on transgender issues, including…
mlance
November 29, 2024 9:43 pm - American Briefing News
‘Explore Every Action Necessary’: Here’s How Trump Admin, GOP May Change Fight Against Mexican Cartels
The Trump transition team and congressional Republicans have promised an unprecedented immigration crackdown, which could also include a novel approach to combating drug cartels. President-elect Donald Trump’s immigration platform includes a number of hardline measures, such as resuming construction on the U.S.-Mexico border wall, reviving the Remain in…
mlance
November 29, 2024 2:24 am - American Briefing News
America’s First Thanksgiving Proclamation Was About The Constitution — And Not Everyone Liked It
When the first Congress met at Federal Hall in New York City in 1789, there were three days in September that saw a telling sequence of events. On Thursday, Sept. 24, the House of Representatives finalized the language for what the states would…
mlance
November 29, 2024 2:21 am - American Briefing News
‘This Is A Great Day’: Here’s What Legal Experts Got Right About Trump’s Criminal Cases
President-elect Donald Trump faced four criminal cases during the 2024 election, but his risk of imprisonment has vanished after securing the presidency, confirming predictions from several legal experts. Special counsel Jack Smith’s two federal cases against Trump are no longer moving forward, and the president-elect’s two…
mlance
November 29, 2024 2:17 am - American Briefing News
America’s Largest And Most Expensive DEI Program Is About To Go Up In Flames
The University of Michigan’s (UM) multi-million dollar diversity, equity and inclusion (DEI) program may soon be dismantled. The university’s board of regents has reportedly asked UM president Santa Ono “to defund or restructure” the DEI office amid growing criticism and public pressure, according to emails shared on X.
mlance
November 27, 2024 9:30 pm - American Briefing News
GOP Reps To Grill Top Biden Officials On Natural Gas Exports Pause
The House Oversight and Accountability Committee is bringing in top Biden administration energy officials for a Dec. 4 hearing on the administration’s decision to pause approvals for certain new liquefied natural gas (LNG) export facilities. Subcommittee on Economic Growth, Energy Policy, and Regulatory Affairs Chairman Pat Fallon, a Republican from…
mlance
November 27, 2024 9:28 pm - American Briefing News
Help Is Coming For The Border

Army Command Sgt. Maj. John W. Troxell, Senior Enlisted Advisor to the Chairman of the Joint Chiefs of Staff, visits numerous points along the southern border during a trip to Arizona, May 29, 2019. Marine Corps Sgt. Maj. Paul McKenna, North American Aerospace Defense Command and U.S. Northern command senior enlisted leader, Marine Corps Sgt. Maj. Aaron G. McDonald, Joint Task Force North command senior enlisted leader, and Army Command Sgt. Maj. Alberto Delgado, U.S. Army North (Fifth Army) Command Sgt. Maj., joined Troxell on the trip. (DoD Photo by U.S. Army Sgt. James K. McCann)
The border crisis reached unprecedented levels under the Biden-Harris administration. Still, there is finally a light at the end of the tunnel: President-elect Donald Trump will soon begin his second term as president of the United States, and he has made it clear that on day one …
mlance
November 27, 2024 9:26 pm - American Briefing News
‘If You Want Something, Come Get Some’: Tom Homan Responds To Threats Live On Air
Former U.S. Immigration and Customs Enforcement (ICE) Director and incoming “border czar” Tom Homan said Wednesday on Fox News that he would not be intimidated by those seeking to harm him, following bomb threats and “swatting” calls directed at President-elect Donald Trump’s cabinet picks. Incoming White House press secretary Karoline…
mlance
November 26, 2024 8:49 pm - American Briefing News
‘Wasteful Subsidies’: Ramaswamy Slams Biden Admin For Rushing $50 Billion Chip Subsidies Ahead Of Trump Takeover
Vivek Ramaswamy took to social media Tuesday to criticize the Biden administration for its rapid allocation of $50 billion in chipmaking subsidies. In a post on X, previously known as Twitter, Ramaswamy shared a Politico interview where Commerce Secretary Gina Raimondo detailed her plans to speed up the…
mlance
November 26, 2024 8:48 pm - American Briefing News
Court Of Appeals Ends Trump’s Legal Battle Over Classified Documents In Florida
The 11th Circuit Court of Appeals accepted Special Counsel Jack Smith’s request Tuesday to terminate the legal proceedings against President-elect Donald Trump in the Florida documents retention case. The ruling dismisses charges related to Trump’s possession of classified documents after his presidency, The Hill reported. Smith advocated…
mlance
November 26, 2024 8:46 pm - American Briefing News
Feds’ Lifeline To Small Businesses Might Be ‘Death Knell’ For ‘Some’ Companies
A Federal Reserve program aimed at bolstering small and medium-sized businesses may now be financially burdening some of them, experts told the Daily Caller News Foundation. The Fed introduced the Main Street Lending Program (MSLP) in 2020, which provided loans to small and mid-sized businesses that were impacted…
mlance
November 25, 2024 10:04 pm - American Briefing News
‘The Guy Has Real Charisma’: Ex-CNN Analyst Speculates About Whether Donald Trump Jr. Will Run For White House In 2028
Former CNN political analyst Chris Cillizza speculated Monday on the possibility of Donald Trump Jr. running for president in 2028, suggesting he could inherit his father’s “political movement.” President-elect Donald Trump picked Vice President-elect JD Vance as his running mate after Trump Jr. strongly advocated for…
mlance
November 25, 2024 9:43 pm - American Briefing News
GOP Rep Looks To Thwart Potential Trump Resistance In Department Of Labor
Republican North Carolina Rep. Virginia Foxx wrote to Department of Labor (DOL) Acting Secretary Julie Su Monday to ensure the agency continues to perform casework during the remainder of President Joe Biden’s lame duck term. During the 2016-2017 transition from former President Barack Obama to President-elect Donald Trump, the DOL slowed or ceased…
mlance
November 25, 2024 9:42 pm - American Briefing News
Democrats Set To Elect New Leader Following Trump’s Inauguration
The Democratic National Committee (DNC) will elect its new chair and other key officers on Feb. 1 at the party’s winter meeting in National Harbor, Md. Members of the DNC are preparing to appoint a new leadership team. The roles to be filled include chair, vice chairs, treasurer, secretary and…
mlance
November 24, 2024 10:03 pm - American Briefing News
Trump Has The Tools To Purge DEI From Every Facet Of Government — Here’s How He Might Use Them
After four years of the Biden administration embedding diversity, equity and inclusion (DEI) into nearly every aspect of government, the incoming administration has plenty of work ahead to make good on President-elect Donald Trump’s promise to uproot “woke” ideology. The Trump administration will need to address everything from agency hiring…
mlance
November 24, 2024 10:00 pm - American Briefing News
Poll Shows Majority Of Voters Approve Trump’s Transition
A newly released CBS News poll on Sunday showed that a majority of voters (59%) approve of how President-elect Donald Trump is handling the transition of power. Just weeks before Election Day, rhetoric from both corporate media and Democrats intensified against Trump, with Vice President Kamala Harris …
mlance
November 24, 2024 9:57 pm - American Briefing News
It’s Time To Downsize The DEI Headcount At HHS
Under President Joe Biden, the Department of Health and Human Services (HHS) has created a “diversity, equity and inclusion” (DEI) infrastructure of dizzying complexity. The agency’s 2025 budget request contains the word “equity” 829 times, adding onto a dollar total that is already impossible to fully tabulate. HHS employed at…
mlance
November 23, 2024 9:48 pm - American Briefing News
The West Is Playing With Fire In Ukraine
As wars tend to do, the battle over Ukraine continues to escalate. It was reported this week that North Korean soldiers in the conflict total 10,000 thus far and that Russia has rewarded Pyongyang by sending its excellent air defense systems to the Korean Peninsula in exchange. Last month, the National…
mlance
November 23, 2024 9:45 pm - American Briefing News
Laken Riley Murderer’s Trial Shows How Woke District Attorneys Endanger Our Communities
The decision by Georgia’s woke district attorney to prioritize political agendas over justice for 17-year-old Laken Riley is an affront to accountability. Instead of delivering the justice her grieving family deserves, Deborah Gonzalez, a George Soros-backed prosecutor, argued that pursuing harsher penalties could lead to “collateral consequences to undocumented defendants.” This twisted…
mlance
November 23, 2024 9:37 pm - American Briefing News
This Country Is Cracking Down On Social Media Speech — And It’s Not China Or Russia
Social media censorship and arrests over posts online have become prevalent in the United Kingdom (U.K.), especially in 2024. The U.K. has been at the forefront of implementing digital speech laws that regulate what civilians can and cannot say online, with prohibitions on what it defines as “hate…